Nearly a decade ago, more than 80 global organizations came together to sign the London Declaration on Neglected Tropical Diseases, to control, eliminate, or eradicate at least ten of the diseases by 2020. Progress has been made on “NTDs” (neglected tropical diseases), but they still affect nearly one billion people, reinforcing poverty in low-resource regions.
 UC Berkeley Professor of Bioengineering Dan Fletcher has been working to accelerate NTD diagnosis and treatment with a device so ubiquitous it’s probably in your hand: a smartphone. Fletcher, with a group of interdisciplinary scientists from in and outside his lab, have steadily proved that smartphone cameras can be repurposed as microscopes to identify disease-causing pathogens, such as the bacteria that cause tuberculosis and the parasites that cause River Blindness.
UC Berkeley Professor of Bioengineering Dan Fletcher has been working to accelerate NTD diagnosis and treatment with a device so ubiquitous it’s probably in your hand: a smartphone. Fletcher, with a group of interdisciplinary scientists from in and outside his lab, have steadily proved that smartphone cameras can be repurposed as microscopes to identify disease-causing pathogens, such as the bacteria that cause tuberculosis and the parasites that cause River Blindness.
“Phones have enormous imaging and processing capabilities,” explained Fletcher at a recent faculty salon of the Blum Center, where he serves as chief technologist. “Our work has been to harness these consumer electronics to do the complex imaging and image interpretation tasks. We add lenses and automation to the mobile devices; then we capture, geotag and upload the image data we collect, giving us greater information about the spread of disease.”
By removing the need for a laboratory and highly trained technicians to perform image-based processing, the Fletcher Lab’s CellScope, as the family of devices is called, can dramatically expedite NTD treatment by enabling patients to receive rapid, low-cost, and highly accurate diagnoses, even in remote regions. Fletcher and others believe the device has enormous potential to contribute to NTD elimination efforts, because while the drugs to treat NTDs already exist–an efficient, systematic, and affordable diagnosis and treatment method does not.
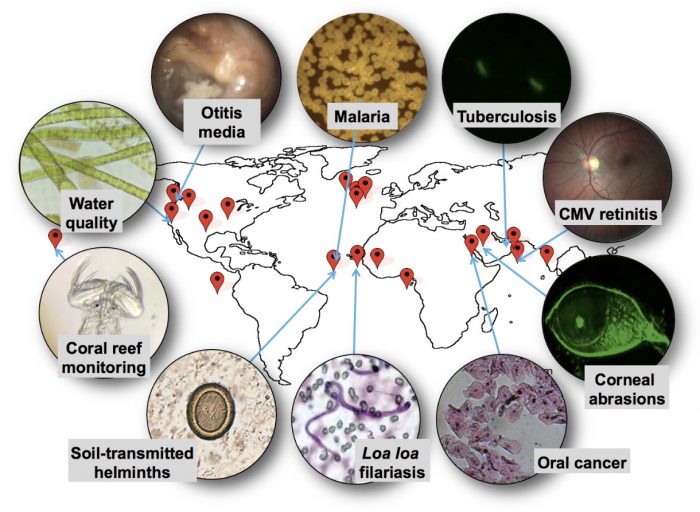
“Thanks to the London Declaration, major pharmaceutical companies have pledged to contribute the drugs needed to treat many NTDs,” said Fletcher. “CellScope will help us tackle the missing ingredient: the way to identify those in need. For effective treatment and monitoring, we must know who’s infected, where they are, and whether there is reemergence. That’s where technology comes in.”
The latest version of CellScope, called LoaScope, has proven effective in Cameroon, where it is being used to combat a NTD called River Blindness or Onchoceriasis. Endemic in Sub-Saharan Africa, Onchoceriasis is a severely debilitating disease that results in human blindness. Although the antiparasitic drug, Ivermectin, is readily available, treatment via mass drug administration has been halted in some regions due to serious adverse effects caused by co-infection with a worm call Loa loa. Treatment in Loa-endemic regions is complex because if Ivermectin is administered to someone who is simultaneously infected with Loa Loa, the drug can lead to fatal complications.
To safely relaunch mass drug administration of Ivermectin, Fletcher and a team of medical professionals from NIH in the U.S., IRD in France, and CRFilMT in Cameroon launched a pilot project in Okola District of Cameroon, with funding from the Bill and Melinda Gates Foundation. Local healthcare workers collected small blood samples from patients and inserted them into the LoaScope to measure the number of parasites; if the number was below a certain safety threshold, then the patient was treated with Ivermectin. Patients with parasite loads above a certain threshold did not receive Ivermectin to reduce the possibility of complications from treatment.

With this approach, 96 percent of the 16,000 people examined in the Okola District pilot study had Loa loa levels below the safety threshold and were treated for River Blindness, while only 2 percent had to be excluded because of Loa loa levels that put them at risk of complications from treatment (the remaining 2 percent could not receive Ivermectin for other reasons such as pregnancy or illness). Findings from the Cameroon pilot were published in 2017 in the New England Journal of Medicine.
The Cameroon Ministry of Health approved the study and backed the use of the LoaScope technology to restart mass administration of Ivermectin for control of River Blindness. Prior to the pilot study, a public health education campaign to encourage citizens to seek safe diagnosis with LoaScope was carried out–including public testing of the Minister of Health with the LoaScope.
Although the Cameroon pilot was a success, the next challenge is scaling the technology so that the millions of people living in Loa-endemic regions can benefit from safe treatment of River Blindness with ivermectin.
“The problem is no longer that we don’t have the tools. We now do,” said Fletcher. “The problem right now is the funding, manufacturing, and distribution gap–how do we best scale this device and get it to the people who need it?”
Funds are needed to mass produce the device, establish a support infrastructure, and develop an IT system. Yet the cost benefits could be significant. For example, currently the cost of a single capillary for the LoaScope is $2; if the process was automated, the cost would decrease to just 30 cents. A similar reduction in cost of the device is possible.
In addition to River Blindness, the Fletcher Lab has identified five other NTDs for which CellScope could be applied. Schistosomiasis is the next disease the lab hopes to test and treat. Like River Blindness, schistosomiasis is a worm-based disease, but the parasites that cause it are detected in urine or stool samples. Thus, instead of taking a blood sample, CellScope would be adapted to analyze filtered urine and stool that is loaded into the same capillary and imaged with the same device.
“The basic technology–a mobile microscope with image processing capabilities–is already there, and with a few modifications we can adapt it to new diseases,” said Fletcher. “Increasing access to high-quality disease diagnosis is beginning to be within reach for low-resource settings with technologies such as these. But the same challenge remains: scaling.”